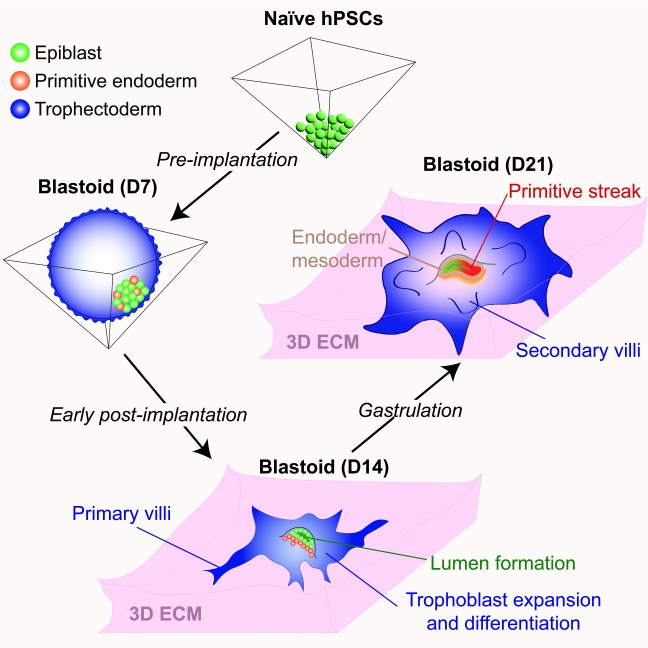
Our paper on extended human blastoid culture was published today in Cell Stem Cell. In a transformative advance, several groups have reported that naive human pluripotent stem cells (hPSCs) can form blastocyst-like structures (also known as “blastoids”) that model the human pre-implantation embryo. However, the extent to which blastoids can recapitulate defining features of post-implantation development remained unexplored. In this study, we optimized the conditions for blastoid generation from naïve hPSCs and investigated their capacity for extended culture on thick 3D extracellular matrices, which better mimic the physical environment of the human endometrium compared to flat surfaces. We developed an experimental methodology that supports human blastoid culture for up to day 21 (D21), including the formation of complex embryonic and placental structures. By performing a detailed single cell transcriptome analysis at three distinct time points (D7, D14, and D21), we benchmarked our model system to human embryos at pre-implantation, early post-implantation, and early gastrulation stages.
3D-cultured human blastoids display several molecular and morphogenetic hallmarks of early post-implantation development, including lumenogenesis of the epiblast compartment, rapid expansion and diversification of trophoblast lineages, and robust invasion of extravillous trophoblast cells by D14. Extended blastoid culture resulted in the formation of a primitive streak-like structure, as evidenced by the localized activation of TBXT (Brachyury) by D18. Blastoids maintained until D21 acquired a single cell transcriptome profile that closely resembled that of a gastrulating human embryo analyzed at Carnegie Stage 7. This included the emergence of blastoid primordial germ cells, definitive endoderm, and various mesodermal lineages, and a diverse array of extraembryonic cell types, including blastoid amnion, cytotrophoblast, extravillous trophoblast, extraembryonic mesoderm, syncytiotrophoblast, and yolk sac endoderm. Thus, the 3D-cultured human blastoids described herein model embryonic and extraembryonic development from pre-implantation to early gastrulation stages, offering a continuous and integrated in vitro model system of early embryogenesis.
Congratulations to Rowan, our collaborators in the Dietmann and Zhou labs, and the entire team!
Note: The generation of integrated models of human development in our laboratory is entirely supported by private foundation grants and does not involve federal funding from the National Institutes of Health.